What are the ideal chemical levels for a pool?
Maintaining a balanced pool is essential for a clean, safe, and inviting swimming environment. Understanding the ideal ranges for pool chemicals is key to achieving this balance and ensuring the longevity of your pool and its equipment. This guide provides a comprehensive overview of the ideal chemical ranges for your pool, helping you keep your water clear and your pool in top shape.
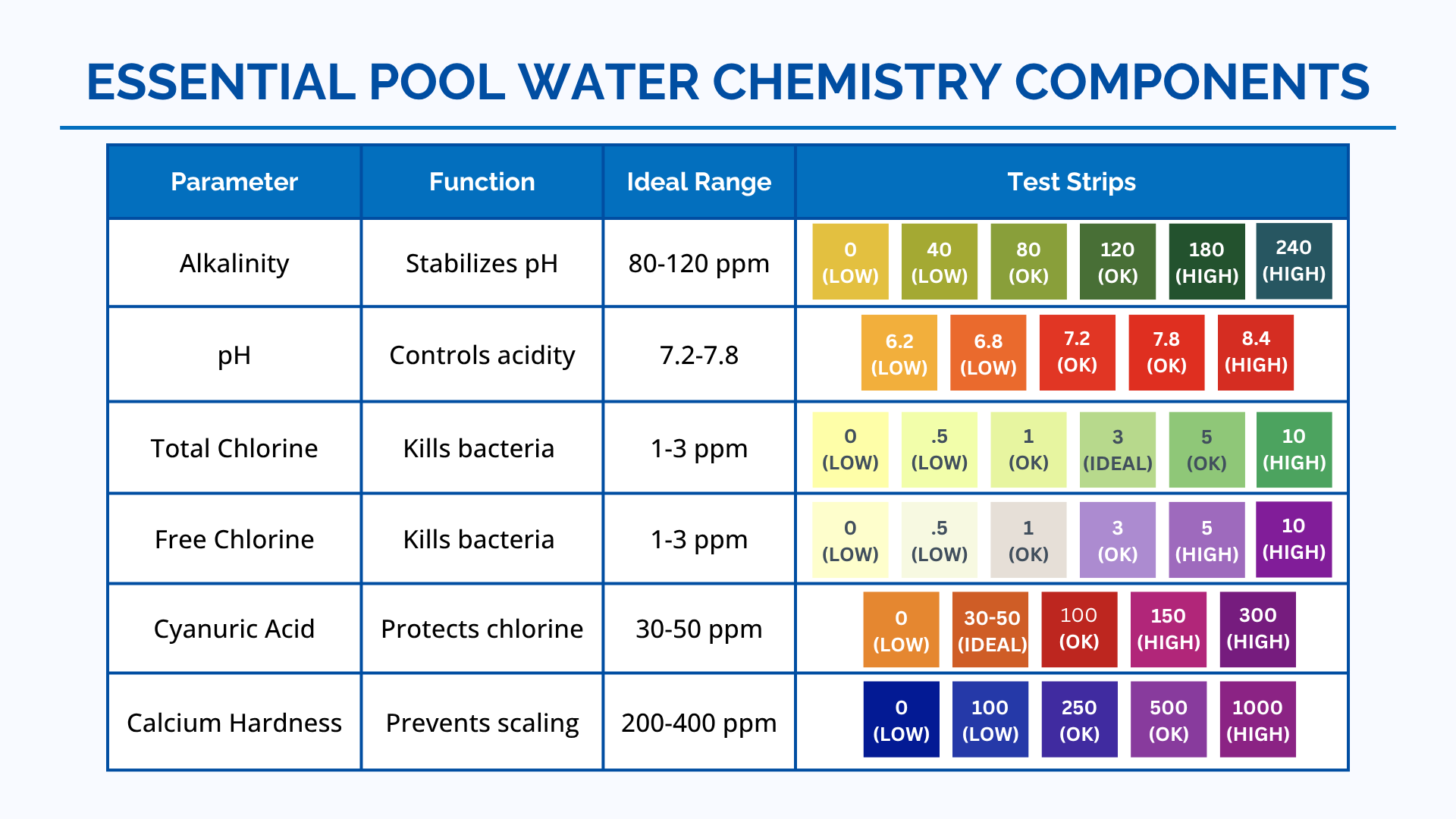
pH Levels
Ideal Range: 7.4 to 7.6
Why It Matters: The pH level measures how acidic or alkaline your pool water is. Keeping the pH within the ideal range ensures that chlorine works effectively, minimizes skin and eye irritation, and prevents damage to pool equipment and surfaces.
How to Maintain: Use pH increasers (sodium carbonate) to raise the pH and pH decreasers (sodium bisulfate or muriatic acid) to lower it. Regularly test the pH level using pool test strips or a digital tester.
Free Chlorine
Ideal Range: 1.0 to 3.0 ppm
Definition: Free chlorine is the chlorine available in the pool water that is not yet combined with contaminants. It actively works to disinfect the pool by killing bacteria, viruses, and algae.
Why It Matters: Free chlorine ensures effective sanitization and prevents waterborne illnesses.
How to Maintain: Use chlorine tablets, granules, or liquid chlorine to adjust levels. Regularly check chlorine levels with a pool test kit and adjust as necessary.
Combined Chlorine
Ideal Range: Below 0.2 ppm
Definition: Combined chlorine, or chloramines, forms when free chlorine reacts with organic substances like sweat and body oils. It is less effective at sanitizing and can cause unpleasant odors and irritation.
Why It Matters: High levels of combined chlorine indicate contamination and reduced effectiveness of chlorine.
How to Maintain: Perform regular shock treatments to break down combined chlorine and restore free chlorine levels. Ensure effective filtration.
Total Chlorine
Ideal Range: 1.0 to 3.0 ppm
Definition: Total chlorine is the sum of free chlorine and combined chlorine. It represents the total amount of chlorine in the pool water.
Why It Matters: Helps understand overall chlorine levels but does not directly indicate sanitizing effectiveness.
How to Maintain: Subtract free chlorine from total chlorine to find combined chlorine (Total Chlorine - Free Chlorine = Combined Chlorine). Address high combined chlorine with shock treatments.
Alkalinity
Ideal Range: 80 to 120 ppm
Why It Matters: Total alkalinity acts as a buffer to stabilize pH levels. Proper alkalinity helps prevent sudden pH changes and maintains water balance, protecting your pool surfaces and equipment.
How to Maintain: Use alkalinity increasers (sodium bicarbonate) to raise levels and decreasers (muriatic acid) to lower them. Regular testing will help you keep alkalinity within the ideal range.
Calcium Hardness
Ideal Range: 200 to 400 ppm
Why It Matters: Calcium hardness measures the concentration of calcium in your pool water. Proper levels prevent water from becoming too corrosive or scaling, both of which can damage pool surfaces and equipment.
How to Maintain: Use calcium increasers (calcium chloride) to raise levels if they are too low. Regularly test calcium hardness and make adjustments as needed.
Cyanuric Acid (Stabilizer)
Ideal Range: 30 to 50 ppm
Why It Matters: Cyanuric acid helps stabilize chlorine, protecting it from being degraded by sunlight. This ensures that chlorine remains effective for longer periods.
How to Maintain: Use stabilizer products to increase levels. Avoid overusing stabilizers, as excessive cyanuric acid can reduce chlorine effectiveness.
Total Dissolved Solids (TDS)
Ideal Range: Below 1,500 ppm
Why It Matters: TDS measures the total concentration of dissolved substances in pool water. High TDS levels can affect water balance and the efficiency of pool chemicals.
How to Maintain: Regularly replace a portion of the pool water to reduce TDS levels. Monitor TDS with a test kit and address any imbalances promptly.
Copper
Ideal Range: 0.0 to 0.2 ppm
Why It Matters: Copper can enter pool water through algaecides or plumbing. Excessive copper can lead to staining of pool surfaces and greenish discoloration of water.
How to Maintain: Test for copper using a specific pool test kit. If levels are high, use a metal sequestrant or remover to address the issue and prevent staining. Ensure proper maintenance of your pool filtration system to avoid metal buildup.
Iron
Ideal Range: 0.0 to 0.3 ppm
Why It Matters: Iron can enter pool water through well water or metal equipment. High iron levels can cause stains on pool surfaces and contribute to water discoloration.
How to Maintain: Test for iron using appropriate pool test kits. Use metal sequestrants or stain removers to manage and reduce iron levels. Regular maintenance and proper water source management can help prevent iron contamination.
Tips for Maintaining Ideal Pool Chemical Ranges
- Regular Testing: Consistently test your pool water using reliable test strips or digital testers to ensure all chemical levels are within the ideal ranges.
- Consistent Maintenance: Regularly add the necessary chemicals to keep your pool water balanced. Follow manufacturer instructions and adjust as needed based on test results.
- Professional Assistance: If you are unsure about balancing your pool chemicals or facing persistent issues, consult a pool maintenance professional for expert advice.
- Seasonal Adjustments: Be mindful of changes in pool usage, weather conditions, and seasons, as these factors can impact chemical levels and require adjustments.
By maintaining the ideal chemical ranges for your pool, you ensure a clean, safe, and enjoyable swimming environment. Regular testing and adjustments will help you keep your pool in optimal condition year round.


